79.7g of blue vitriol reacts with KI then find the volume of I2 at STP 4CuSO4+4KI=Cu2I2+2K2SO4+I2?
1 Answer
63.4 g is produced. It is not formed as a gas.
Explanation:
The iodine produced will not be a gas under normal conditions so I will calculate the mass produced.
For a start the equation is not balanced. It should be:
Iodine forms in solution with a brown precipitate of copper(I) iodide:
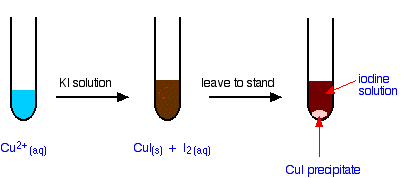
It does not form as a gas at stp.
The equations tells us that 2 moles
Converting moles to grams:
Another example of a very bad question. If the iodine was produced as a gas at stp (which it isn't) then we would reluctantly have to say:
Moles
Assuming the molar volume at stp is 22.4L then :
Volume
This gives option B as the correct answer to an incorrect question.

