Why F2 is dia magnetic ?
1 Answer
Jun 8, 2018
Well the fluorine ATOM,
Explanation:
And in the fluorine molecule, the valence orbitals PAIR up to fill the electronic orbitals of a diatom...
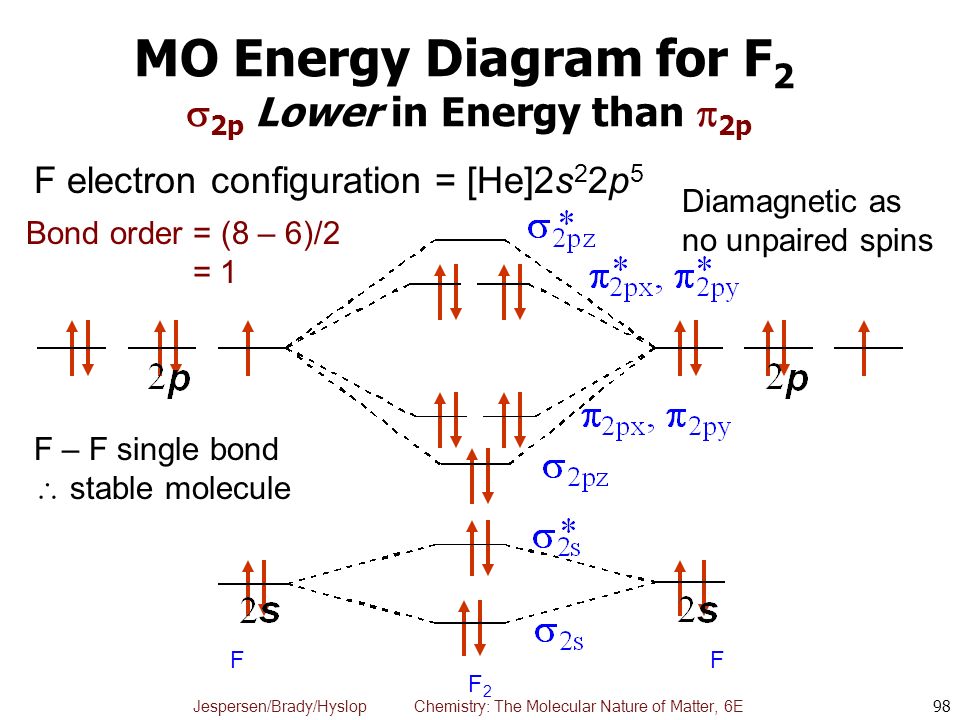
There is ONE NET bonding orbital … depicted in the diagram as

