Rank each of the elements in order of decreasing ionization energy: S, Cl, Br? (Justify)
1 Answer
Jun 11, 2018
Explanation:
Ionization energy is the energy needed to remove one electron from an atom in the gaseous state.
Note that this electron would be a valence electron, or an electron in the outermost energy level/shell, because they're the easiest to remove.
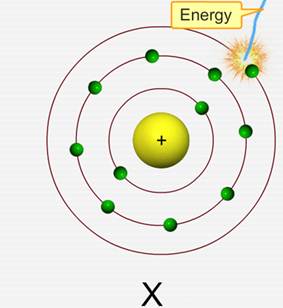
Ionization energy generally depends on the strength of the attraction between the negative valence electron and the positive nucleus. This means that it is affected by two factors:
- How positive the nucleus is. The more protons in the nucleus, the greater the attraction.
- How many energy levels there are. The greater the distance between the valence electron and the nucleus, the less the attraction.
So, keeping this in mind, these are the given elements in the periodic table:
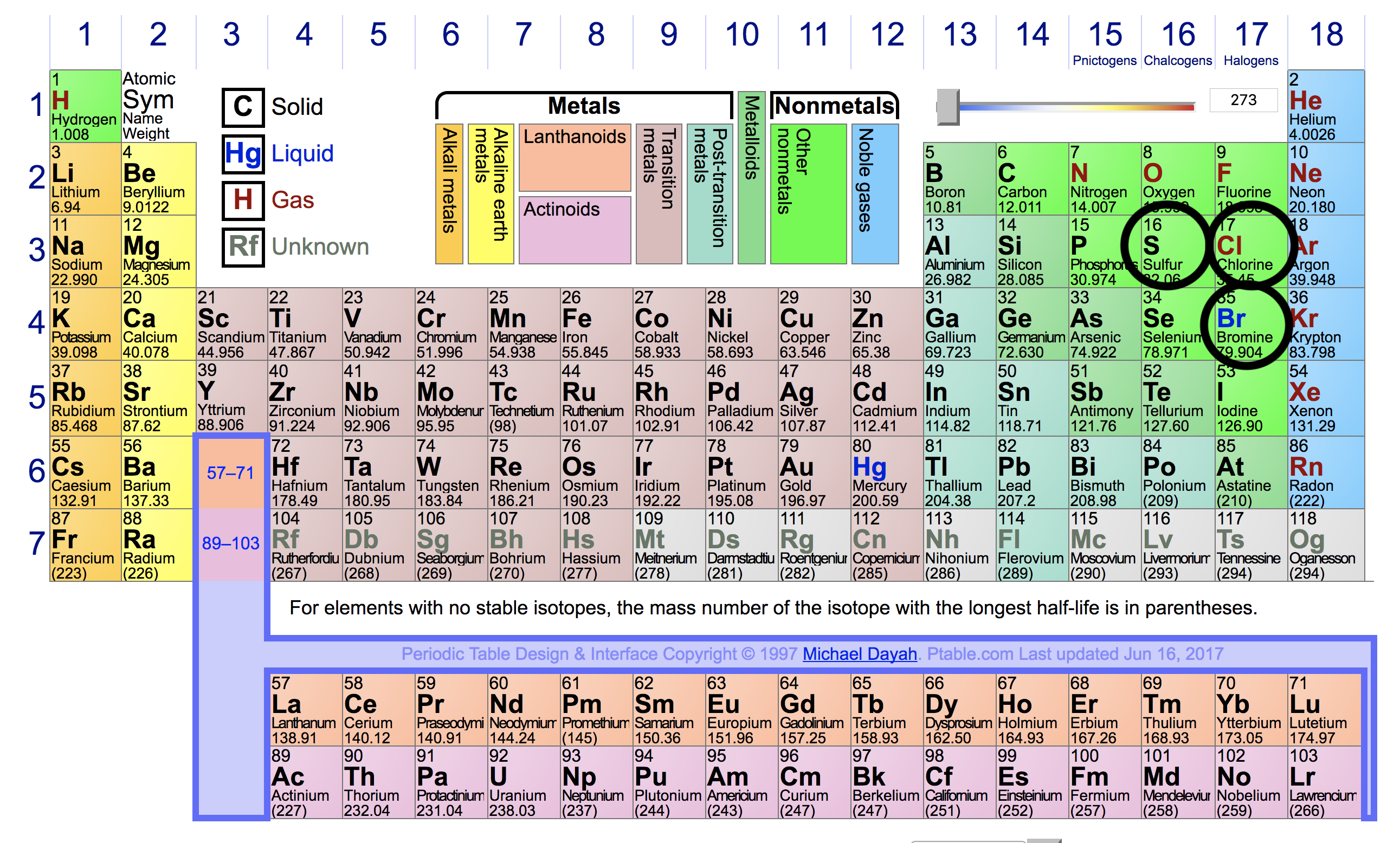
From this, we can know that:
#Cl# will have the greatest ionization energy, because:
Its ionization energy is greater than#S# , because its nucleus is more positive.
Its ionization energy is greater than#Br# , because, while#Br# 's nucleus is more positive,#Br# has one more energy level.#S# will have the smallest ionization energy—it has the least positive nucleus.#Br# 's ionization energy will be greater than#S# , but smaller than#Cl# , because:
Its very positive nucleus makes up for its extra energy level, making its ionization energy greater than#S# .
However, the positive nucleus is not enough to make up for its extra energy level when compared to#Cl# . So, its ionization energy will be smaller than#Cl# 's.

