What covalent compound is P4S5?
1 Answer
Jun 12, 2018
Explanation:
Here is the structure of the compound:-
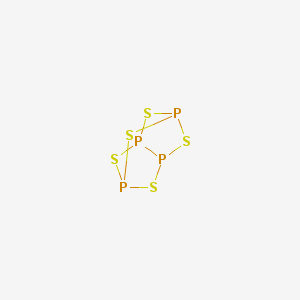
Some properties of the compound:-
Here is the structure of the compound:-

Some properties of the compound:-