What is the ionic radius trend for cations and anions?
some say that it increases towards the lower left corner on the periodic table. But why does Mg have smaller ionic radius than F? That just contradicted with the trend
some say that it increases towards the lower left corner on the periodic table. But why does Mg have smaller ionic radius than F? That just contradicted with the trend
1 Answer
Upon oxidation, would we not immediately predict that the radius of the ion with respect to that of the PARENT atom should DECREASE?
Explanation:
Why? Well examine the reaction....
Now, reasonably, we determine the atomic radius OF ANY SPECIES by the radius of its valence electron. But if we remove that valence electron, then inevitably the radius of the CATION should substantially decrease given that we have removed the shielding effect of an electron, and the nuclear charge contracts the radius of the remaining electrons...
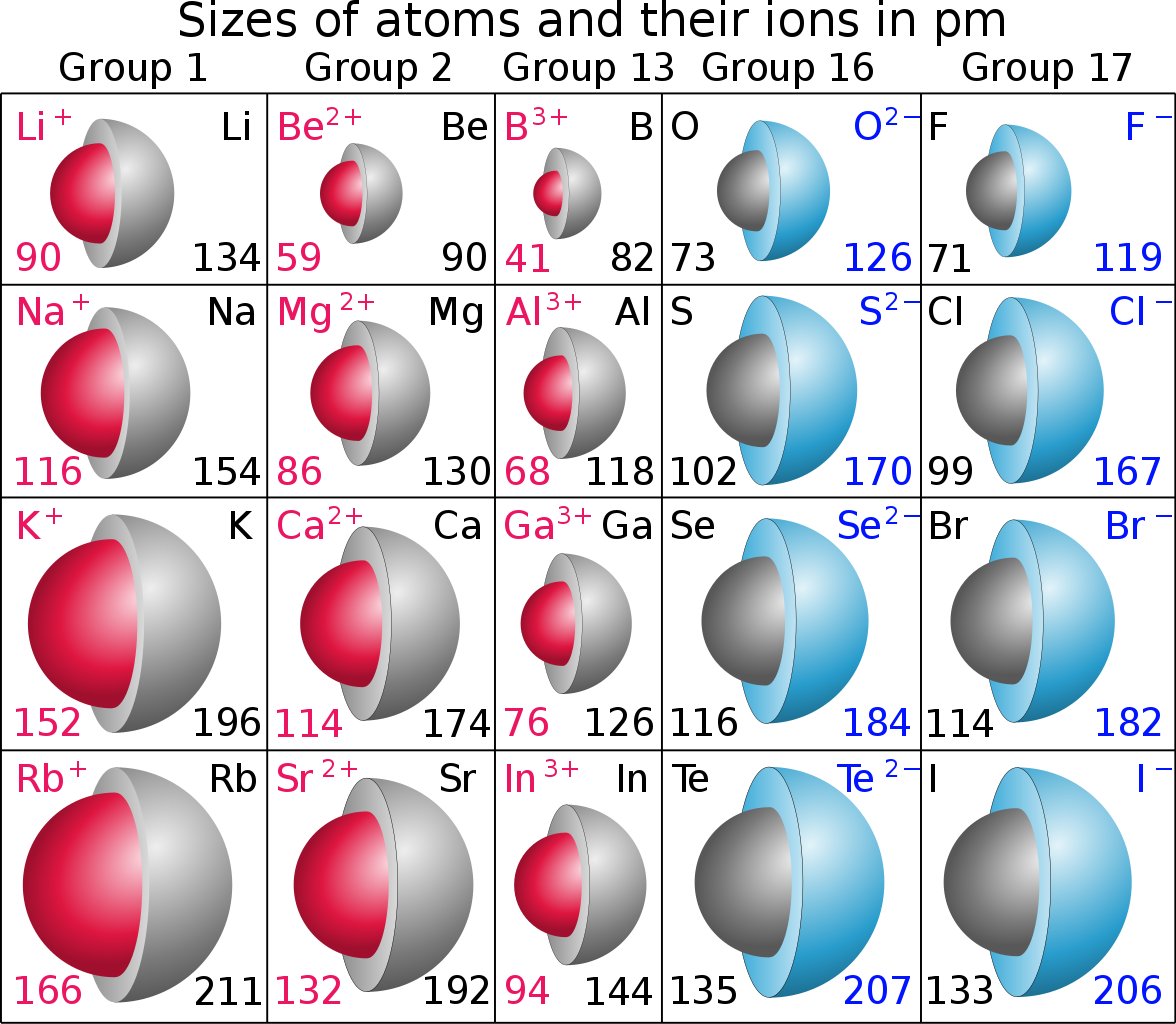
And this effect is CERTAINLY illustrated by the given data; look at the cationic radii with respect to the parent atomic radii (data are in
On the other hand, when an atom is REDUCED....an electron(s) is ADDED to the valence shell, and increased electronic shielding means that the resultant anion is LARGER with respect to the parent atom.
As always, metals are electron-rich species, and TEND to be oxidized..whereas non-metals, high
With respect to your initial question (I finally got there even if I did go off on a tangent!), we compare a 2nd Period non-metal, with a 3rd Period metal...and the Periodic change should be significant with regard to ionic and atomic size. So we really compare apples and oranges here rather than a fairer assessment comparing say fluoride anion, and lithium cation. You happy with this?

