Al2O3+ 6HCl -> 2AlCl3 + 3H2O . We have 3 moles Al2O3 and 14 moles HCl until the balance will react 2 moles Al2O3. What is equilibrium constant?
1 Answer
Jun 18, 2018
1/4 (0.25)
Explanation:
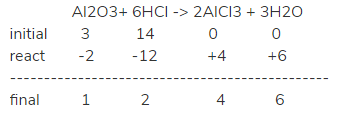
In aqueous solution, we don't have to consider the concentration of water when calculating the equilibrium constant.
K is simply
(we can suppose that there is one liter of water here for better calculating the concentration)

