Spin quantum number of 16th electron of chlorine is?
1 Answer
It is -1/2.
Explanation:
To determine the spin number of an electron, it helps to draw an orbital diagram for chlorine's ground state like so:
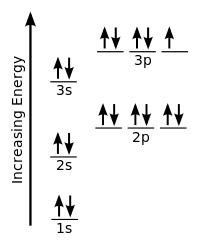 https://commons.wikimedia.org/wiki/File:Electron_configuration_chlorinesvg
https://commons.wikimedia.org/wiki/File:Electron_configuration_chlorinesvg
The up arrow represents an electron with a +1/2 spin number and the down arrow represents an electron with a -1/2 spin number. No two electrons within the same sub shell can have the same spin number.
Thus, to get the spin number of the 16th electron you need to count like so:
1s = 2 electrons
2s= 4 electrons
2p = 7 electrons to fill each sub shell by half
2p= 10 electrons to fill the entire orbital
3s = 12 electrons
3p = 15 electrons to fill each sub shell by half
3p = 17 electrons to fill 2 sub shells
The reason you count like this is to follow Hund's Rule. This rule states that each orbital in a sub shell must be occupied by one electron before a second is added.
Thus, the 16th electron is the 2nd electron in the first 3p sub shell. This electron has a down spin, corresponding to the spin number -1/2.
If you were to just count the electrons from the bottom-up, you would get the 16th electron to be the 2nd electron in the second 3p sub shell. Although this would get the right answer for chlorine, it would not for other elements like oxygen.

