May someone help me?
- The surface tension and viscosity values for diethyl ether, acetone, ethanol, and ethylene glycol are shown here.
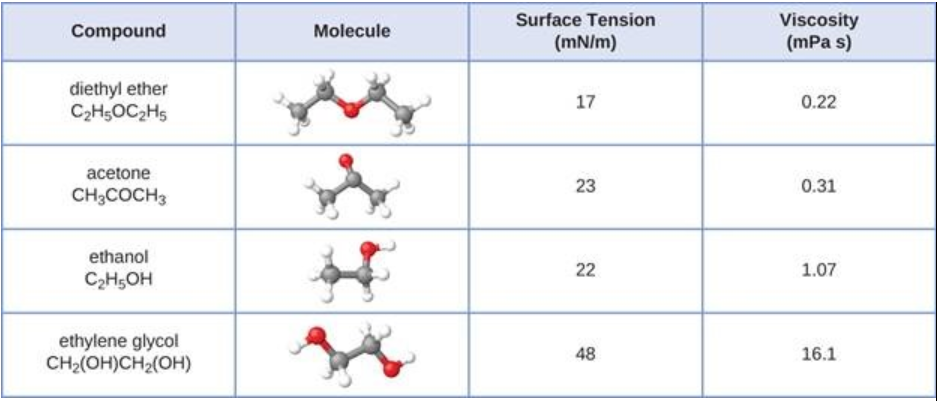
(a) Explain their differences in viscosity in terms of the size and shape of their molecules and their IFs.
(b) Explain their differences in surface tension in terms of the size and shape of their molecules and their IFs.
- The surface tension and viscosity values for diethyl ether, acetone, ethanol, and ethylene glycol are shown here.
(a) Explain their differences in viscosity in terms of the size and shape of their molecules and their IFs.
(b) Explain their differences in surface tension in terms of the size and shape of their molecules and their IFs.
1 Answer
Well, to a first approx. viscosity must SOLELY be a function of intermolecular force, i.e. hydrogen-bonding or polarity, and NOT to dispersion forces....
Explanation:
Why? Well the longest molecule, diethyl ether, HAS the lowest viscosity. The molecule with the MOST hydroxyl groups, ethylene glycol, has the HIGHEST viscosity.
We would ASSUME that the molecule with the MOST hydroxyl groups has the greatest degree of intermolecular force, and this proposition is supported by the data. Ethylene glycol has highest surface tension, and viscosity, and this clearly reflects the degree of intermolecular hydrogen bonding. On the other hand, acetone, and diethyl ether, while somewhat polar, DO NOT engage in hydrogen-bonding, because their hydrogen atoms are NOT bound to a strongly electronegative atom such as oxygen.
Had the melting and boiling of the compounds been given (and perhaps it should have been for a chemistry question), the boiling point would increase in the same order...i.e. the more intermolecular force of attraction, the greater the boiling point. You should quote the boiling points of the molecules in your answer (and of course you will have to look these up).

