Why do all metabolic reactions release heat, such as ATP hydrolysis?
1 Answer
Because it is the most efficient way of living.
Explanation:
Reactions that release energy (heat) are called exothermic reactions.
These reactions do not require a large input of energy in order to get any product.
Reactions that absorb energy are called endothermic reactions. These reactions would require a large input of energy to keep them going, which would be wildly expensive for a living organism.
Here is a potential energy diagram for an exothermic reaction:
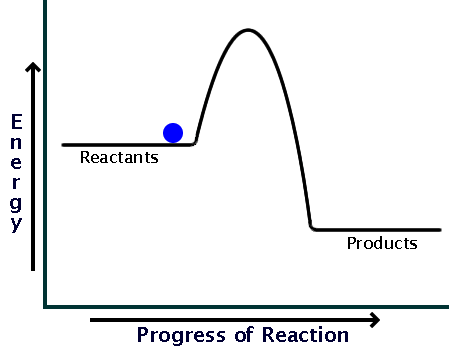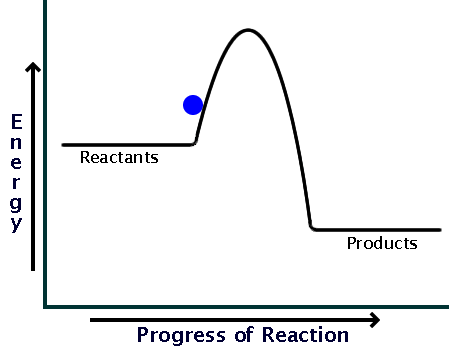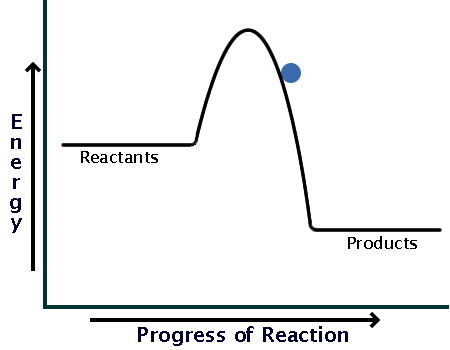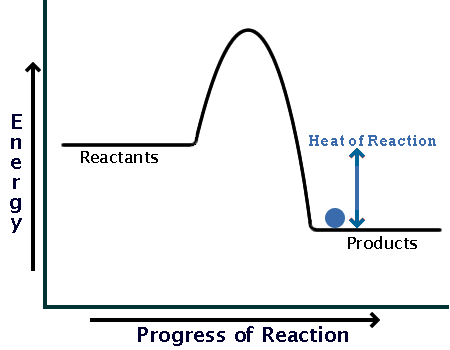
As you can see, the reaction only needs a bit of energy input before it can proceed on its own.
Now here is a potential energy diagram for an endothermic reaction:
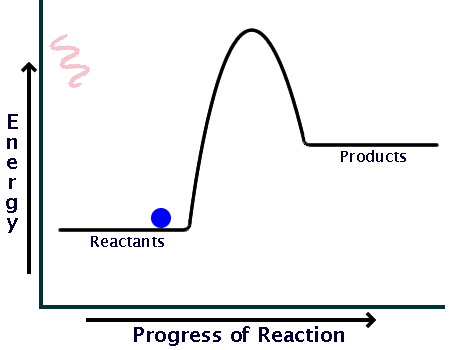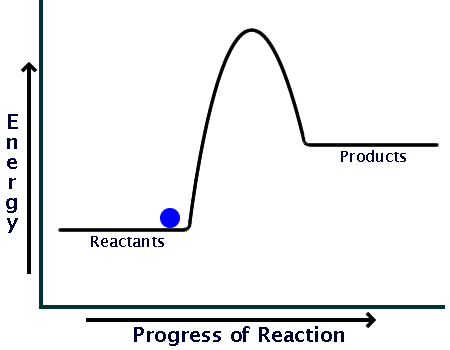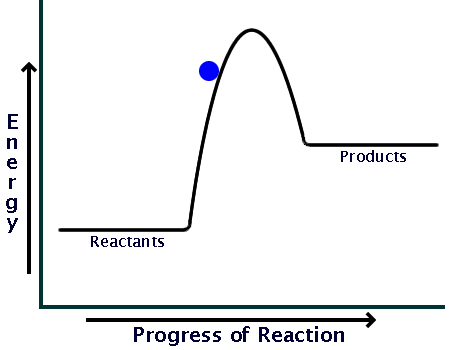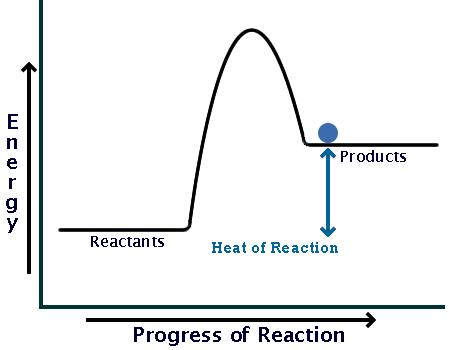
Comparing the sizes of the two hills of the diagrams (activation energy), you can see that endothermic reactions require much larger input than exothermic ones.
Organisms want to conserve as much energy as they can and thus have a metabolism comprised of exothermic reactions instead of endothermic ones.
Hope this helps!

