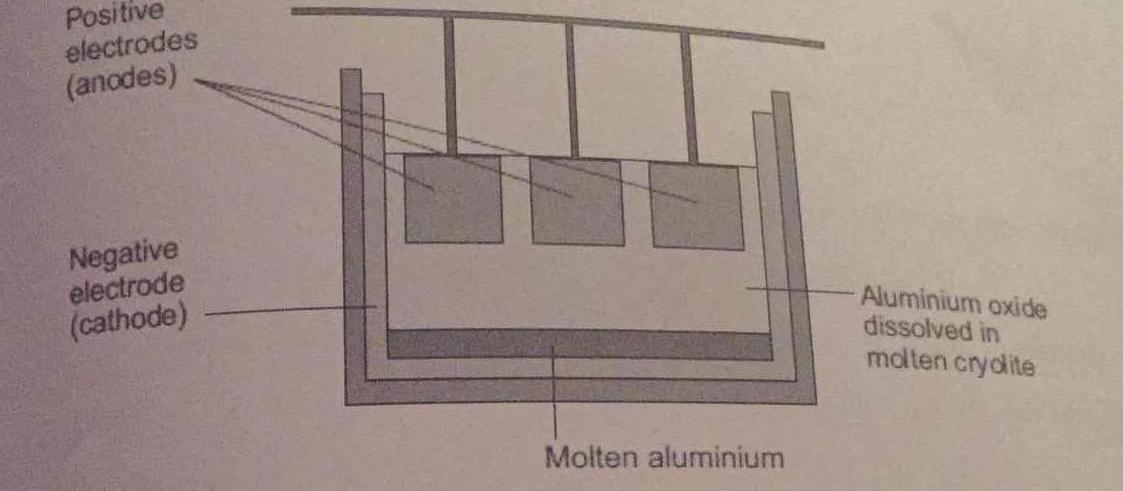
Aluminium is extracted by electrolysis, as shown in the figure. Why can aluminium not be extracted by heating aluminium oxide with carbon?
1 Answer
Jun 28, 2018
more reactive than carbon
Explanation:
aluminium is more reactive than carbon.
carbon is used to displace less reactive metals from their compounds.
this happens since the carbon reacts with the other substance(s) in the compound, and the less reactive metal does not. the result is an isolated metal and a carbon compound.
aluminium is more reactive (higher in the reactivity series) than carbon. it will not be displaced by carbon, meaning that there will be no change in the substances made if aluminium oxide and carbon are heated together.

