If you are wearing wet clothes, and the water evaporates, it cools you down. How does the kinetic theory explain the cooling effect?
1 Answer
Jul 1, 2018
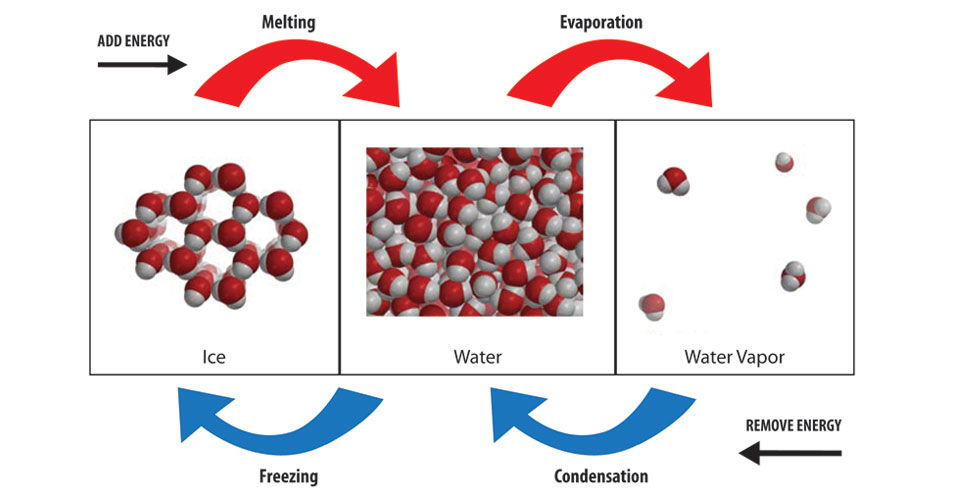
Because evaporation requires energy to excite the molecules of liquid water into gas.
Explanation:
Evaporation requires energy in order to break up intermolecular forces and excite water molecules (fast-moving gas molecules vs. slower moving liquid molecules).
Therefore when the water in the wet clothes evaporates, it is drawing away some heat from your body (the surroundings).
This process is the same in principle as putting a pot of water on the stove to boil it.