Which of the following elements are transition metals: Cu, Sr, Cd, Au, Al, Ge, Co? How can this be determined?
1 Answer
Cu, Cd, Au, Co
Explanation:
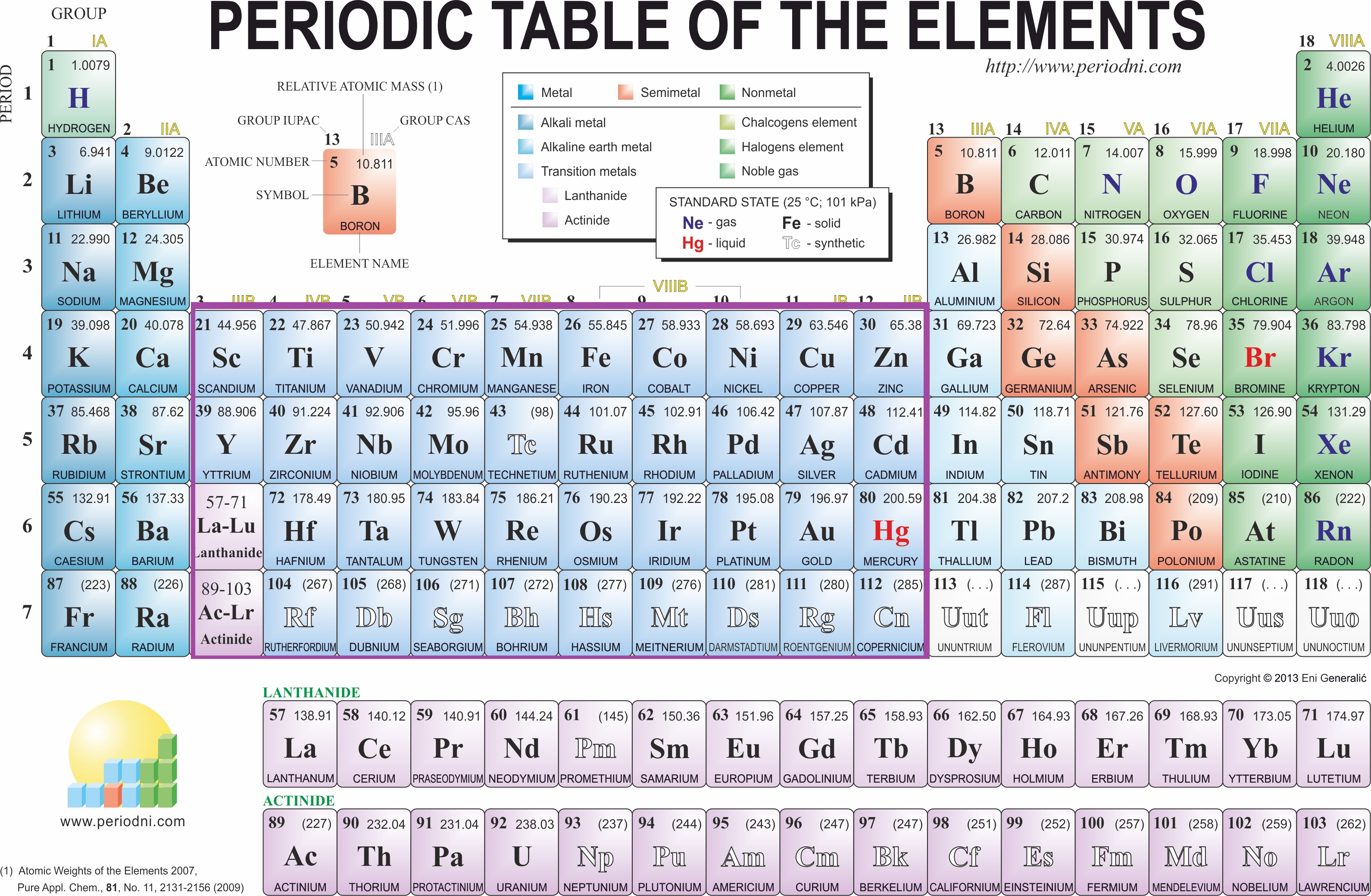
To determine which of these are transition metals using the Periodic Table:
Let's take a look at the Periodic Table.
I put a box around them for visual purposes.
So, first we have Cu. Cu falls within groups 3-12, therefore we can classify it as a transition metal.
Sr is in group 2. Sr is therefore classified as an Alkali Earth Metal, and is not considered a transition metal.
Cd is in group 12. Therefore, it is classified as a transition metal because it falls into our box. The same is said for Au.
Al is in group 13 and is classified as a metal. You may be tempted to say that it's a metalloid, but it is actually a metal.
Ge is a metalloid in group 14.
Co is in group 9, which means that it is a transition metal.

