What is Haber process?
2 Answers
This is dinitrogen fixation....
Explanation:
...the which we may represent as...
The industrial process is carried out under very high pressures and temperatures...and it is only fairly recently that convincing model systems have been developed that represent the fixation of dinitrogen gas at a metal centre, and its reduction to ammonia and hydrazines...
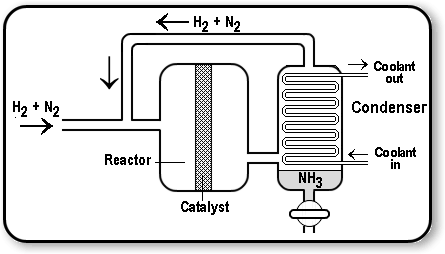
An industrial way to manufacture ammonia.
Explanation:
The Haber process is a common process used to manufacture ammonia from hydrogen and nitrogen. Nitrogen is obtained from the air, while hydrogen is obtained from natural gas.
The chemical equation is:
An iron catalyst is used in the process, and the procedure is carried out at a temperature of around
Ammonia is used to manufacture chemicals, plastics, pesticides, and more.
 https://chemstuff.co.uk/academic-work/a-level/the-haber-process/
https://chemstuff.co.uk/academic-work/a-level/the-haber-process/


