The structure of linalool, a compound with a floral scent used in perfumes, How many pi bonds are shown in the structure?
 d396qusza40orc.cloudfront.net
d396qusza40orc.cloudfront.net
 d396qusza40orc.cloudfront.net
d396qusza40orc.cloudfront.net
1 Answer
Jul 10, 2018
Explanation:
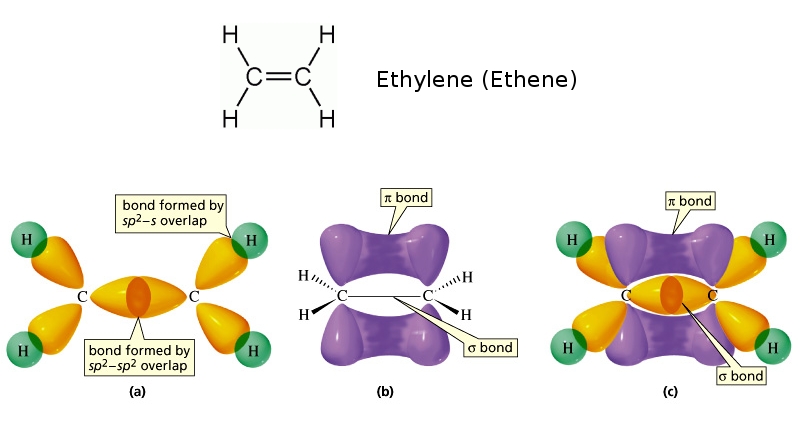
There are two types of carbon-carbon covalent bonds:
sigma (sigma) bonds due to the head-on/axial overlapping of atomic orbitalspi (pi) bonds as a result of the side-way/lateral overlapping ofp orbitals
All carbon-carbon single bonds
Sigma bonds and pi bonds combine to form covalent bonds of orders higher than one. For example, each carbon-carbon double bond contains
- one
sigma sigma bond, and - one
pi pi bond.
as seen in the diagram
 https://brilliant.org/wiki/sigma-and-pi-bonds/
https://brilliant.org/wiki/sigma-and-pi-bonds/
The organic molecule shown in the question contains two carbon-carbon double bonds (shown in red). As a result, this molecule has two
Created with Google Drawings

