Analysis of gaseous bromine vapourised in a mass spectrometer shows that it consists of 51% of Br-79 and 49% of Br-81...?
- In the mass spectrometer ionizing process, what charge will each bromine atom have?
- Sketch a mass spectrogram for the vapourised bromine atoms
- Explain how many peaks it has and why.
- What would be the mass value for each peak?
- In the mass spectrometer ionizing process, what charge will each bromine atom have?
- Sketch a mass spectrogram for the vapourised bromine atoms
- Explain how many peaks it has and why.
- What would be the mass value for each peak?
1 Answer
- Preferably
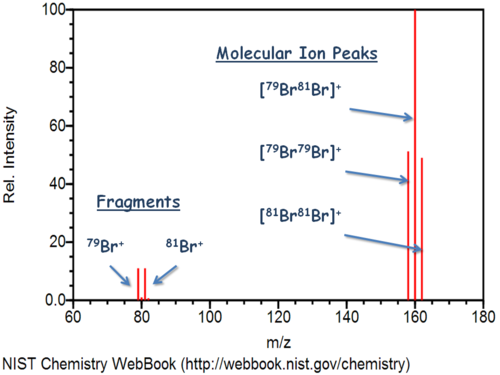
+1 for all atoms. - Five discrete peaks, one for each isotopic atom, and one for each isotopic molecule.
- Percent abundance is indicated by their relative heights.
 https://upload.wikimedia.org/
https://upload.wikimedia.org/
Explanation:
The ionizer in an "electron impact" mass spectrometer would knock an electron off of each bromine atom.
It would preferably remove only one electron from each atom because it is rare that the same particle gets ionized twice.
Ions with a single positive charge can be directly sorted from other ions as they pass through an electric or magnetic field based on their molar mass, as it gives a different
Two isotopes of
-
Two
color(white)(.)_79"Br" make a"Br"_2 molecule of mass number158 ,
which is of mass2 * 78.92 ~~ 157.84 color(white)(l) g * mol^(-1)
This configuration is found inp(79)^2 ~~ 26% of all"Br"_2 molecules -
One
color(white)(.)_79"Br" and onecolor(white)(.)_81"Br" make a"Br"_2 molecule of mass number160 ,
which is of mass78.92 + 80.92 ~~ 159.84 color(white)(l) g * mol^(-1)
This configuration is found in2 * (p(79) * p(81)) ~~ 50% of all"Br"_2 molecules -
Two
color(white)(.)_81"Br" makes a"Br"_2 molecule of mass number162 ,
which is of mass2 * 80.92 ~~ 161.84 color(white)(l) g * mol^(-1) ,
This configuration is found inp(81)^2 ~~ 24% of all"Br"_2 molecules
Reference
"Isotopes of Bromine," https://en.wikipedia.org/wiki/Isotopes_of_bromine


