A half-life of potassium -40 is 1.28X10^9 years. How much of 100.00 g sample will be left after 5.12X10^9 years? .
1 Answer
Jul 25, 2018
The amount left is
Explanation:
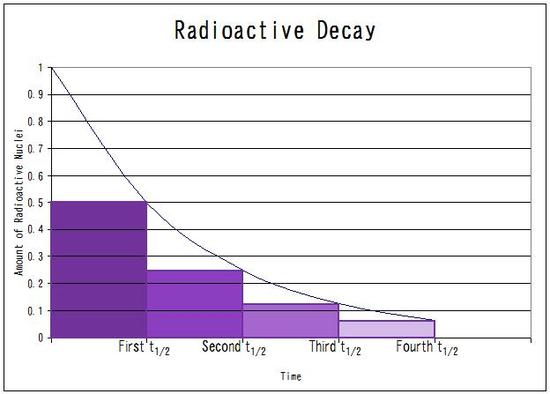
The half -life of
The time is
The initial amount is
Half the amount is left after
That is
In
As
The amount left in

