Why following compound is anti-aromatic?
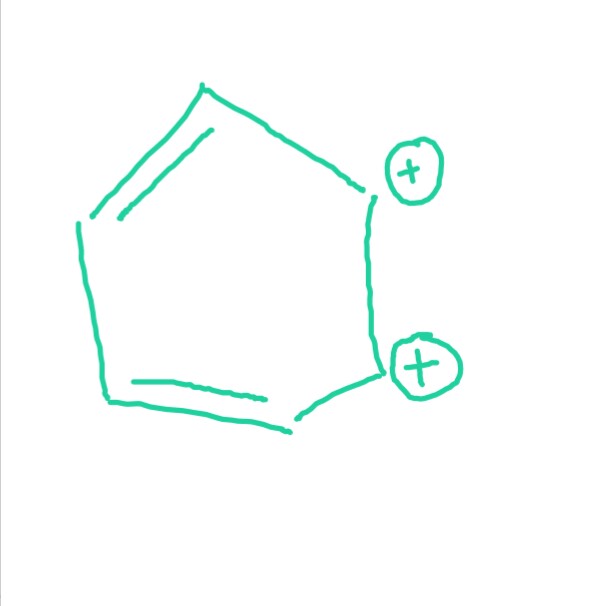
This compound is not even have complete conjugation. Yeah, even though it follows (4n)π electrons rule.
This compound is not even have complete conjugation. Yeah, even though it follows (4n)π electrons rule.
1 Answer
Jul 25, 2018
Well, how many
Explanation:
I count FOUR....and these are delocalized around a six-membered ring. Given this, it is an analogue of cyclobutadiene, and therefore ANTIAROMATIC.
Of course, this would be an high energy species, that I doubt has been isolated....

