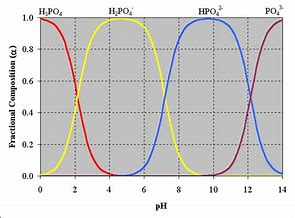
What is the normality of 2M solution of phosphoric acid of an acid base titration?
1 Answer
Aug 1, 2018
Approx.
Explanation:
Phosphoric acid acts as a DIACID in aqueous solution. The solution after addition of excess base should be stoichiometric in
Unless special measures are take only the 2 equiv of protium ion are titratable.