In a vacuum, will a heated solid sublime?
2 Answers
Not necessarily; it depends on the solid. In the case of
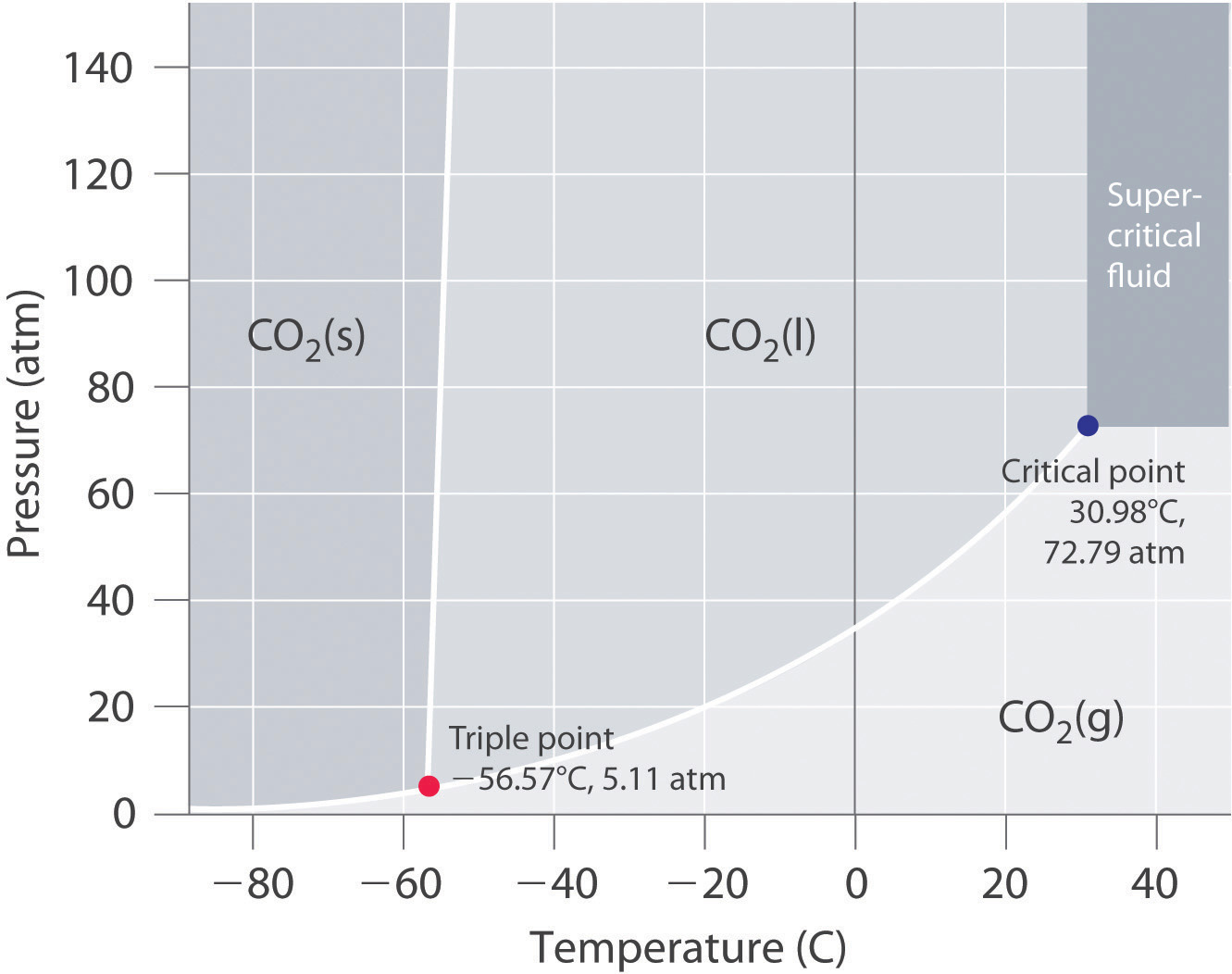
In a phase diagram like this, if you start at
If the liquid-solid coexistence curve starts at a triple point that exists at really low pressures (a vacuum), and if the solid-gas-liquid-gas coexistence curve is really flat, then even in a vacuum, at a high enough temperature, maybe a solid can still exist as a solid and then heat up until it becomes a liquid rather than a gas.
But you have to look at the phase diagram before assuming that a solid turns into a gas upon heating if in a vacuum.
If you are very lucky...
Explanation:
Given a tube furnace, and a turbomolecular pump, MANY inorganic compounds will sublime. And sometimes this includes simple halide salts...that you would normally not think are volatile. The sublimate is usually very pure; however, you must have special apparatus to store the sublimed (or twice-sublimed salt, I kid you not!) salt, and typically this is an inert atmosphere dry box.



