4790 joules of heat are added to 222 grams of water originally at 27.7 C. What is the final temperature of the water?
2 Answers
If I am correct, you will need to know the equation
Explanation:
Here, you are given the values for Q, m and c can be obtained from your textbook. According to mine, the value should be 4.19 J/C.
Note that I let x represent the final temperature. And now, all you have to do is some simple arithmetic and solve for x!
The final answer is 32.8°C
The final temperature is
Explanation:
Use the specific heat equation:
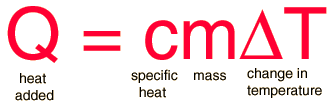
Known
Unknown
Solution
Rearrange the equation to isolate
Divide
Now that
Add


