When does a single replacement reaction occur?
1 Answer
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
Explanation:
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
There are two types of single replacement reactions:
A metal replaces another metal that is in solution:
Example:
A halogen replaces another halogen that is in solution:
Example:
To determine whether a given single replacement will occur, you must use an “Activity Series” table.
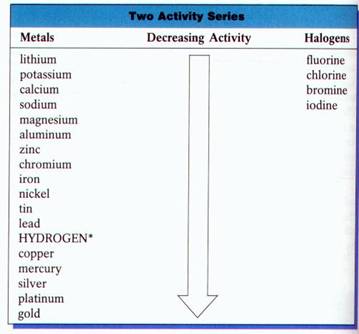
If the metal or the halogen is above the element it will replace based on the activity series, a single displacement reaction will occur.
Examples:
The video below summarizes an experiment conducted to compare the activities of three metals (


