A single displacement reaction is often of the type
#"A + BC → B + AC"#
In this reaction, element #"A"# replaces element #"B"# in the compound #"BC"#.
Here are a few examples of the practical applications of displacement reactions.
Thermite Welding
A mixture of #"Al"# and #"Fe"_2"O"_3# reacts to produce molten iron that is used to join railway rails together.
#"2Al" + "Fe"_2"O"_3 → "Al"_2"O"_3 + "2Fe"#
Al replaces #"Fe"# in #"Fe"_2"O"_3# to form #"Al"_2"O"_3#.

#"CADWELD"^®# process
A mixture of #"Al"# and #"CuO"# is used for creating electric joints between #"Cu"# and steel.
#"2Al" + "3CuO" → "3Cu" + "Al"_2"O"_3#
#"Al"# replaces #"Cu"# in the #"CuO"#.

Steel Making
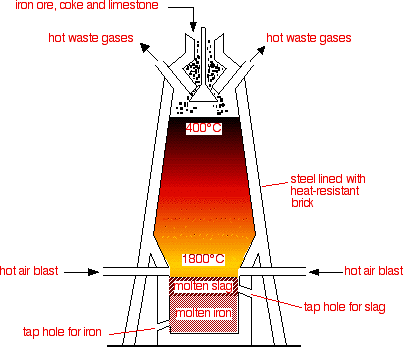
We can extract iron from its ore (#"Fe"_2"O"_3#) by heating it with carbon in giant blast furnaces.
#"3C" + "2Fe"_2"O"_3 → "4Fe" + "3CO"_2#
#"C"# replaces #"Fe"# in #"Fe"_2"O"_3# to form #"CO"_2#.
Extraction of Metals
Heating a mixture of #"Cr"_2"O"_3# and finely divided #"C"# produces metallic chromium.
#"3C" + "2Cr"_2"O"_3 → "4Cr" + "3CO"_2#
#"C"# replaces #"Cr"# in #"Cr"_2"O"_3# to form #"CO"_2# and chromium metal.
Acid Indigestion
We can relieve the distress when the stomach produces too much #"HCl"# by drinking a solution of sodium hydrogen carbonate.
#"HCl" + "NaHCO"_3 → "NaCl" + "H"_2"CO"_3#
The #"H"# in the #"HCl"# displaces the #"Na"# in #"NaHCO"_3# to produce #"H"_2"CO"_3#.




