How do I generate the electron configuration of iron?
1 Answer
An atom in its ground state has the same number of electrons as protons. It is also know as a neutral atom or an uncharged atom.
So, looking at the periodic table, an atom of Iron
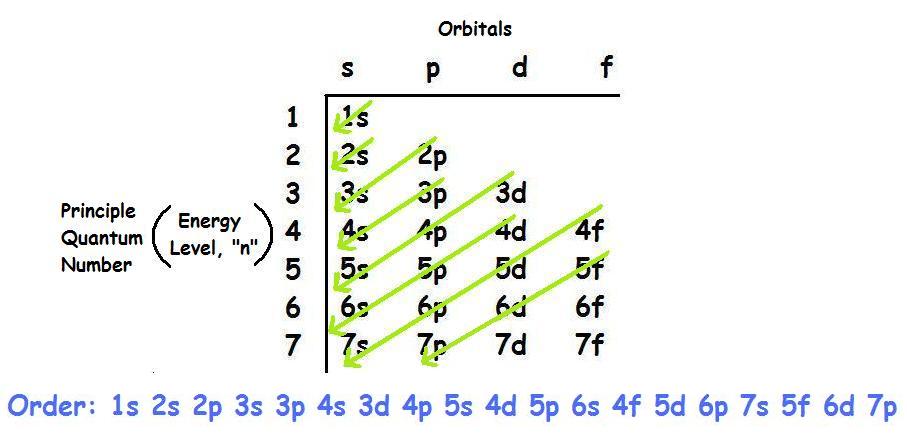
The electrons fill the orbitals closest to the nucleus first and then move out until all of the electrons are accounted for.
The first orbital filled is the
Next, the
Then
So, the electron configuration for Iron would then be:
If you add up the electron numbers (the numbers following the letters
I hope this helps.


