Question #ad27b
1 Answer
In chemistry, a metal is usually an element that is hard, opaque, shiny, malleable, ductile, and a good conductor of heat and electricity. Most of the elements in the Periodic Table are metals.
Solid metals exist as crystals. Their atoms are arranged in an ordered pattern that extends in all directions.
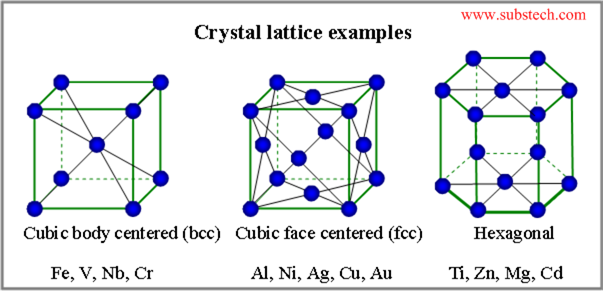
Alloys are substances made by melting a metal and one or more elements together. They have metallic properties and crystallize on cooling.
Some alloys are solution alloys, in which the components are dispersed uniformly.
In a substitutional alloy, the solute atoms replace some of the solvent atoms. Brass is a substitutional alloy of copper and zinc.

In interstitial alloys, the solute atoms fill some of the "holes" (interstices) between the solvent atoms. Carbon steel is an interstitial alloy of carbon and iron.
In summary,
- Solid metals and alloys are crystals.
- Alloys are mixtures of a metal with other metals or nonmetals.
- Metals are pure substances.

