Metals and Nonmetals
Key Questions
-
Answer:
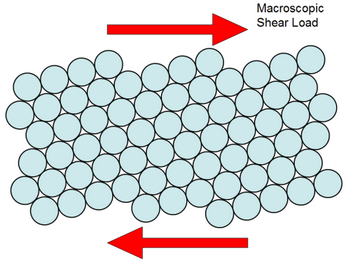
Most metals are malleable because the atoms can roll over each other and retain the structure of the crystal.
Explanation:
Metallic bonds involve all of the metal atoms in a piece of metal sharing all of their valence electrons with delocalized bonds.
This is different from ionic bonding (where no electrons are shared at all) and covalent bonding (where the bonds exist only between two atoms).
A metal that you can hammer into thin sheets is malleable.
Gold, silver, aluminum, iron, and copper are malleable.
Non-malleable metals such as tin will break apart when struck by a hammer.
A metal behaves as an array of metal ions or kernels immersed in a “sea” of mobile valence electrons.
Metallic bonds consist of the attractions of the ions to the surrounding electrons. Metallic bonds are non-directional.
Whenever a metal receives a stress, the position of adjacent layers of metallic kernels shifts.
The atoms roll over each other but the environment of the kernels does not change.
The deforming force just moves the kernels from one lattice site to another.
(from www.continuummechanics.org)