Question #21700
1 Answer
Sep 20, 2014
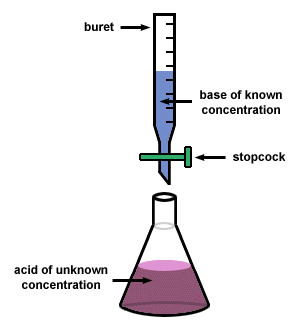
Titration is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte.
- The standard solution is the solution of known concentration.
- The solution of unknown concentration is otherwise known as the analyte.
- The equivalence point is the ideal point for the completion of titration.
- The end point of a titration indicates once the equivalence point has been reached.
For example: