Question #53f3b
1 Answer
Since most solids have periodic arrays of atoms that form crystal lattices, which must imply a degree of symmetry in the arrangement of these lattices.
An ideal crystal can be considered to be a repetition of identical structures in 3D space. Lattices are mathematical points set at specific coordonates in space that can describe the periodicity of such a repetitive structure. Atoms, which are placed in specific lattice points, represent the identical structural units.
All possible lattices can be described by a set of three linearly independent vectors,
Every lattice point can be described by a translation of a vector
where
So, a Bravais lattice represents an infinite array of discrete points that have an arrangement and an orientation that appear exactly the same from whichever point the array is viewed.
14 Bravais lattices are commonly used to classify lattice structures according to basic symmetry groups.
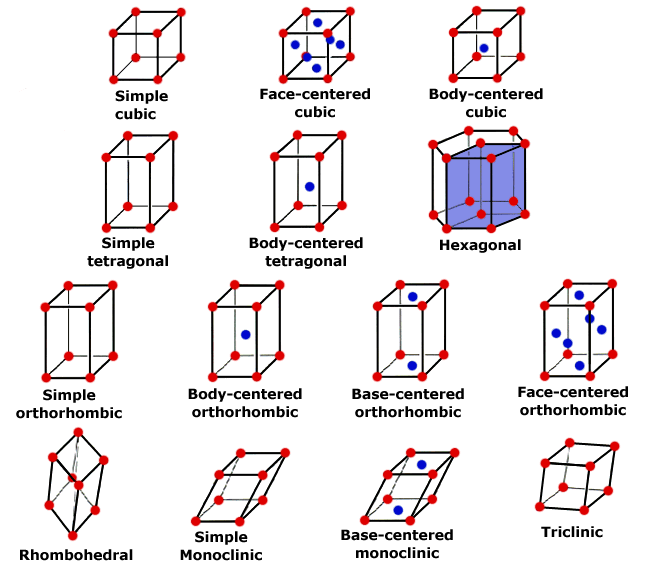 http://www.seas.upenn.edu/~chem101/sschem/solidstatechem.html
http://www.seas.upenn.edu/~chem101/sschem/solidstatechem.html
Each Bravais lattice is obtained by a specific
http://www.seas.upenn.edu/~chem101/sschem/solidstatechem.html
(Here
Here's a video showing all the 14 Bravais lattice structures:

