It is always useful to draw the Lewis structure when trying to determine if a molecule has single, double, or triple bonds.
So, ammonia (NH_3) has a total of 8 valence electrons, 5 from N and 1 from each H atom.
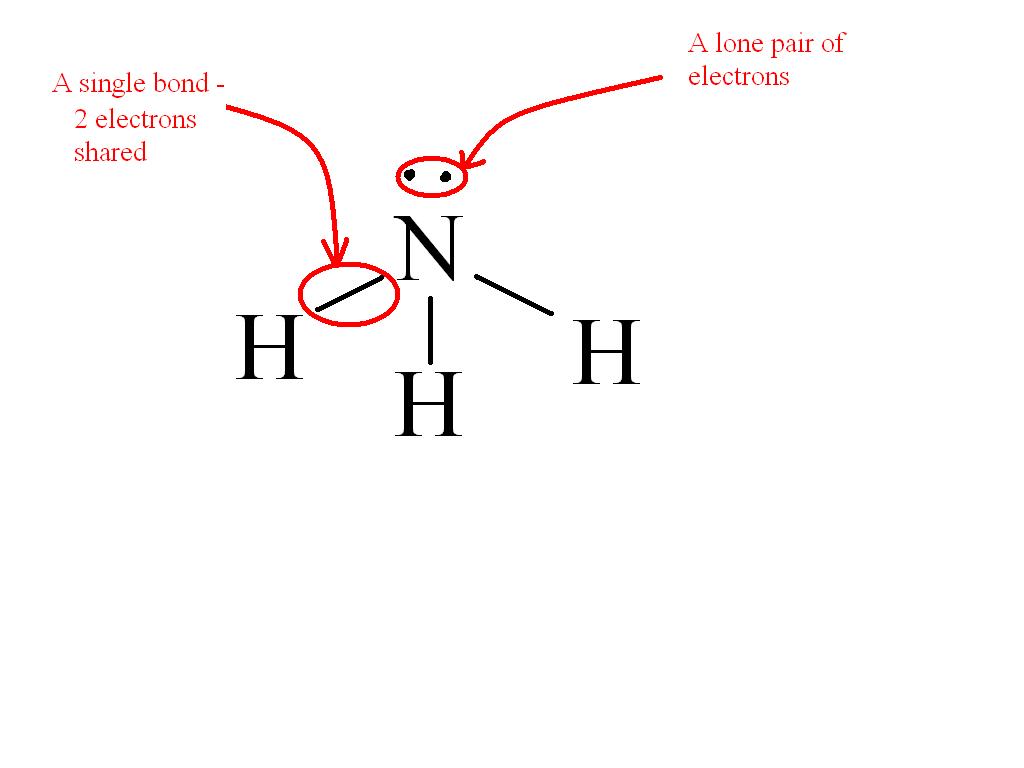
Each H atom bonds with the N in a single bond - 2 electrons shared. The 3 single bonds between N and the H atoms make up for 6 of the valence electrons, the remaining 2 being set as a lone pair around the N atom.
A quick way to determine the type of bonds formed is to take into account the fact that H can only form single bonds, since it only has one orbital filled with one electron; therefore, it only requires one electron more to form a stable doublet configuration.

