Question #1186d
1 Answer
Diatomic oxygen (
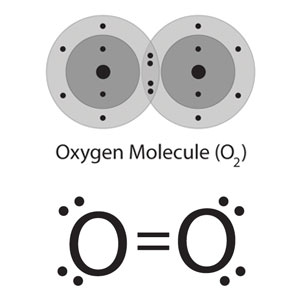
Bond polarity is determined by the difference in electronegativity between the two atoms that form said bond. In
Since both atoms that form the bond have the same electronegativity, there will be no distortion of the electron cloud, which in turns will cause no partial charges to be formed on the molecule.
The thing to remember is that no difference or very little difference in electronegativity means a non-polar bond. Therefore, diatomic oxygen has a non-polar covalent bond.

