Question #d7700
1 Answer
Polyatomic ions are charged chemical species composed of two or more covalently bonded atoms that are considered to act as a single unit.
The nitrite ion, or
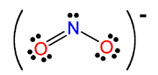
The nitrite ion will form ionic bonds with a variety of positively charged ions (or cations) due to the electrostatic attraction between the negative and the positive charges.
Compounds formed by
Sodium nitrite -
Magnesium nitrite -
Barium nitrit -
Potassium nitrite -
Here's a link to a list containing more compounds formed by the nitrite polyatomic ion
http://www.endmemo.com/chem/common/nitrite.php
A link to an answer on how polyatomic ions bond posted by other contributors:
http://socratic.org/questions/how-do-polyatomic-ions-bond
http://socratic.org/questions/how-do-polyatomic-ions-form-compounds

