How can I learn the valencies of the first #20# elements?
1 Answer
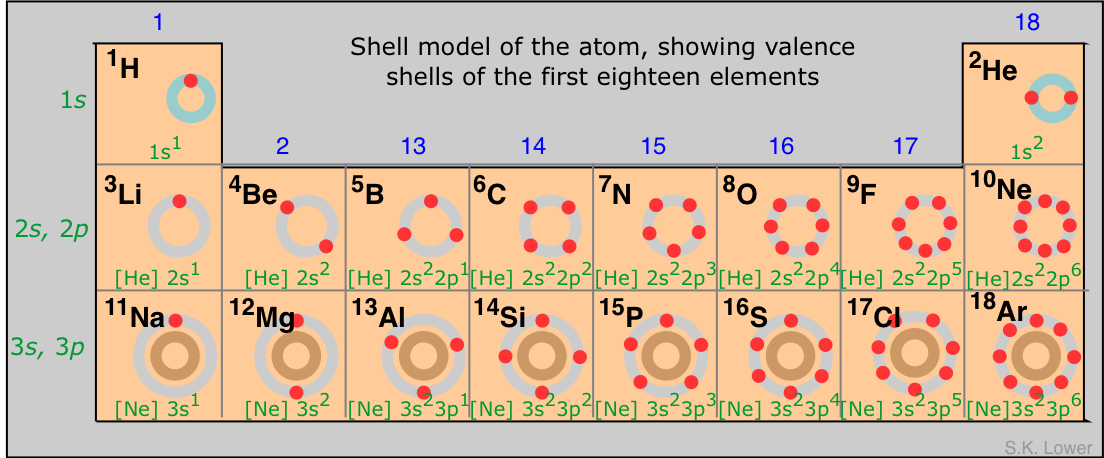
So, the first 20 elements in the periodic table start with H and end with Ca. The quickest way to remember the number of valence electrons is to form a relationship with the number of the group the element is located in.
I'll skip noble gases, since these are elements which have a stable octet, and as a result are usually not a part of chemical reactions.
Starting with the second row:
Group 1 - Li - has 1 valence electron (v.e);
Group 2 - Be - has 2 v.e;
Group 13 - B - has 3 v.e;
Group 14 - C - has 4 v.e;
Group 15 - N - has 5 v.e;
Group 16 - O - has 6 v.e;
Group 17 - F - has 7 v.e;
The group number jumps from 2 to 13 because the first 20 elements are not found in groups 3-12.
Then the pattern repeats itself in the third row. Again,
Group 1 - Na - has 1 v.e;
Group 2 - Mg - has 2 v.e;
Group 13 - Al - has 3 v.e;
Group 14 - Si - has 4 v.e;
and so on.
So, if you can examine a periodic table, the number of valence electrons can be determined if you look at group number.
The digit that represents the units (1 in 1, 3 in 13, 4 in 14, 6 in 16, and so on) is equal to the number of valence electrons.
K and Ca follow the same pattern. For row 4,
Group 1 - K - has 1 v.e;
Group 2 - Ca - has 2 v.e;
So, always think group number

