Question #e4cca
1 Answer
Jul 31, 2015
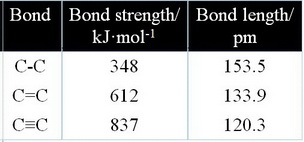
A single bond is the longest and weakest, and a triple bond is the shortest and strongest.
Explanation:
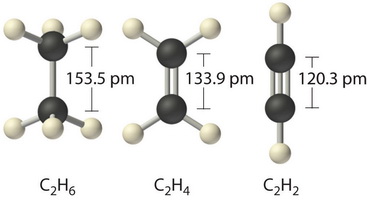
Bond order and bond length are inversely proportional to each other: when bond order is increased, bond length is decreased.
A
(from 2012books.lardbucket.org)
This is a result of the force of attraction between the greater number of pairs of electrons and the two nuclei.
Two pairs of electrons can pull the nuclei closer together than one pair, and three pairs of electrons can pull the nuclei closer than two pairs.
Stronger forces of attraction mean greater bond strengths, so a distinct pattern emerges.
- As the bond strength
#color(red)("IN")# creases, the bond length#color(red)("DE")# creases. - As the bond strength
#color(red)("DE")# creases, the bond length#color(red)("IN")# creases.