Question #a254b
1 Answer
Electronegativity is used to express the ability of an atom that's part of a molecule to attract electrons to itself.
Bond polarity is determined by examining the Pauling scale electronegativity values of the two atoms. The difference between these values will determine the predominant type of bond between the respective atoms.
Here are the electronegativity values for the elements in the periodic table
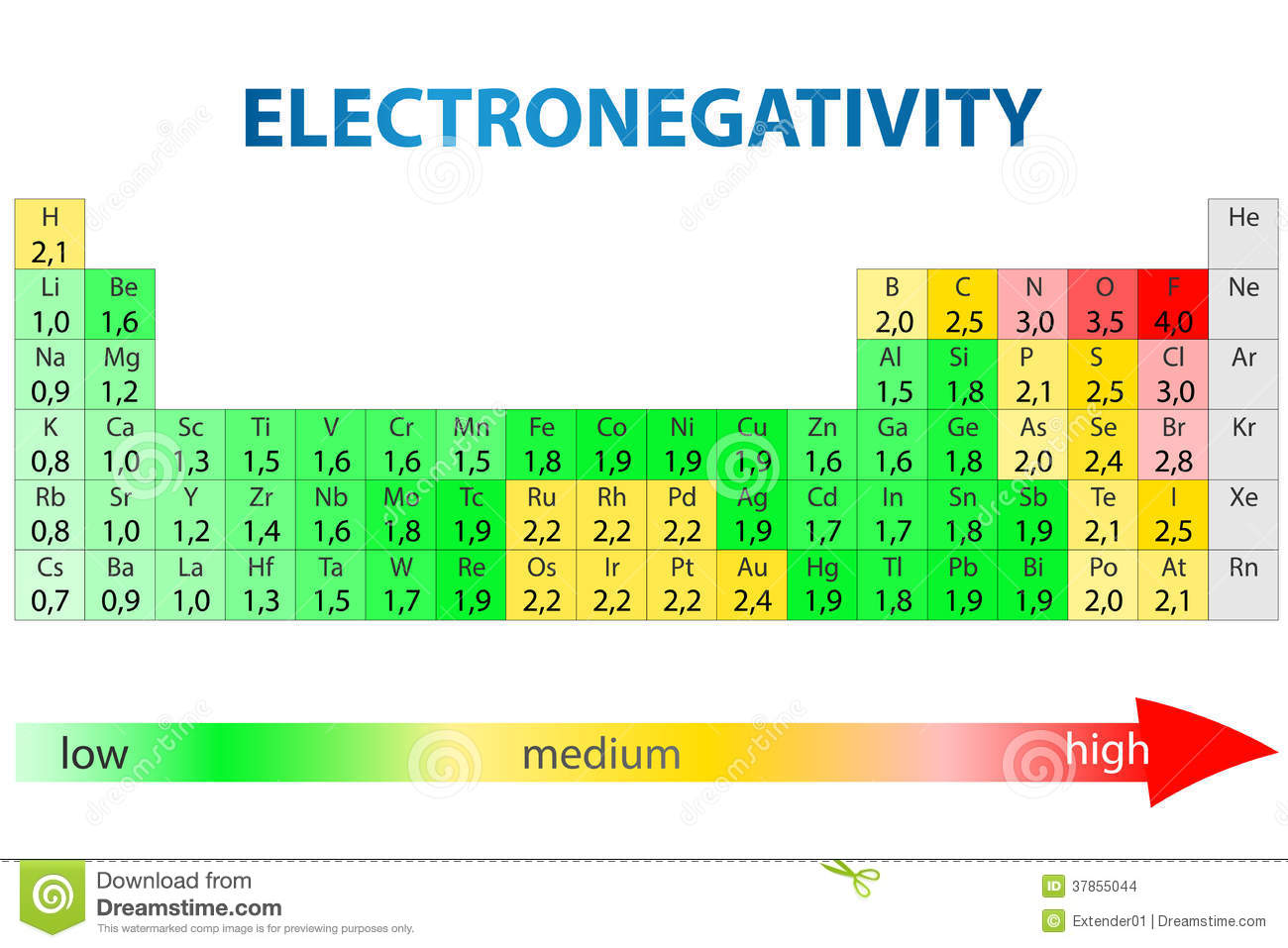
You basically have three types of dominant bond character: covalent bonds, polar covalent bonds, and ionic bonds. The difference in electronegativity values of two atoms bonded together will determine what type of bond they form.
In order for two atoms to form a covalent bond, the difference in electronegativity between the atwo atoms must be less than 0.5. A covalent bond, sometimes called a nonpolar covalent bond, implies equal sharing of the two bonding electrons by the two atoms.
For instance, the
#EN_C - EN_H = 2.5 - 2.1 = 0.4#
Other examples of nonpolar bonds are diatomic molecules such as
For polar covalent bonds, the difference in electronegativity values varies between 0.5 (such as in the
Another classic example of a polar covalent bond is the
#EN_O - EN_H = 3.5 - 2.1 = 1.4#
Ionic bonds occur when the complete transfer of electrons takes place; the bonding electrons are no longer shared between the two atoms, the more electronegative of the two will "take" the bonding electron from the other atom.
In order for abond to be considered ionic, the difference in electronegativity must be greater than 2.0. A classic example for ionic compounds is sodium chloride, or
#EN_(Cl) - EN_(Na) = 3.0 - 0.9 = 2.1#
Another important thing to remember is that, for electronegativity differences that range between 1.6- 1.7 and 2.0, the difference between a polar covalent bond and an ionic bond will be made by the type of atoms that form said bond.
Like I've said,
#EN_(Br) - EN_(Na) = 2.8 - 0.9 = 1.9#
This happens because you have a metal bonded to a nonmetal.
So don't forget to look at what atoms form the bond when dealing with electronegativity values greater than those set as threshold for polar covalent bonds (1.6 - 1.7).

