Question #bd6c7
2 Answers
Sodium (Na) is an alkali metal. It belongs to group 1, and all the elements in group 1 have one valence electron.
Sodium has an electron configuration of:
The valence electron is the one electron located in the 3s sublevel.
Here is a video to help with this concept:
video from: Noel Pauller
Actually, soidum's group number in the periodic table does match its number of valence electrons - soidum is in group 1 and has exactly 1 valence electron.
As you know, valence electrons are the electrons located in the outermost shell of an atom. If you're familiar with electron configurations, you can easily see that this is the case by examining the number of electrons sodium has in its outermost shell.
Since sodium's atomic number is 11, a neutral sodium atom will have a total of 11 electrons surrounding its nucleus. Its electron configuration is
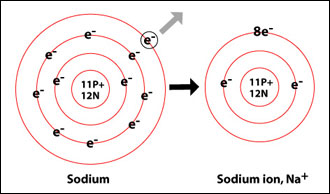
So, sodium's outermost shell, which is sometimes referred to as the valence shell, is 3s. Since this shell is occupied by just one electron, that's how many valence electrons neutral sodium will have
Notice that the sodium cation is formed when that single valence electron is removed from the 3s shell, which allows sodium to have a full octet.


