Why do two fluorine atoms bond together?
1 Answer
Two fluorine atoms bond together to form the fluorine molecule because both those atoms can obtain a full octet by the sharing of two electrons.
Since fluorine is in group 17 of the periodic table, which means it has 7 valence electrons, it only needs one more to complete its octet - 8 electrons in its valence shell.
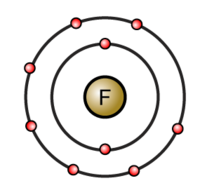
When two fluorine atoms come together, they each share one of their 7 valence electrons to form a nonpolar covalent bond. A nonpolar covalent bond implies that both electrons that form the bond between the fluorine atoms are shared equally.
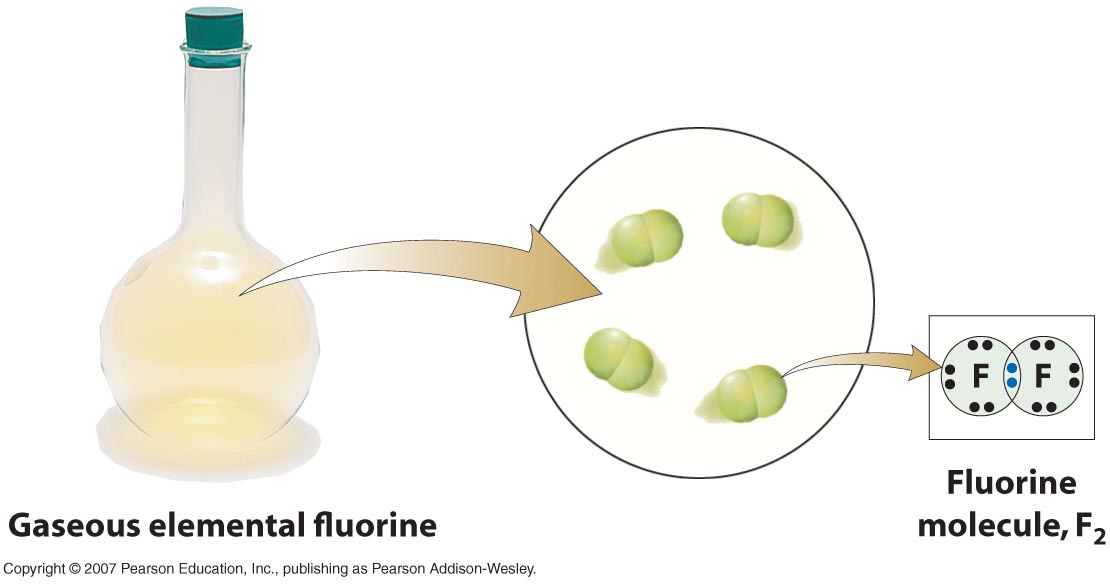
When electrons are shared equally, they spend the same amount of time on both atoms that form the bond, that is why the fluorine molecule, or