Which of the following substances are insoluble in water?
1) sodium chloride
2) silver nitrate
3) potassium sulfate
4) copper(II) hydroxide
1) sodium chloride
2) silver nitrate
3) potassium sulfate
4) copper(II) hydroxide
1 Answer
That would be copper (II) hydroxide, or
You will need to become familiar with the solubility rules, because that will help you determine what compounds are soluble and what compounds are insoluble in water.
Here's what these rules look like
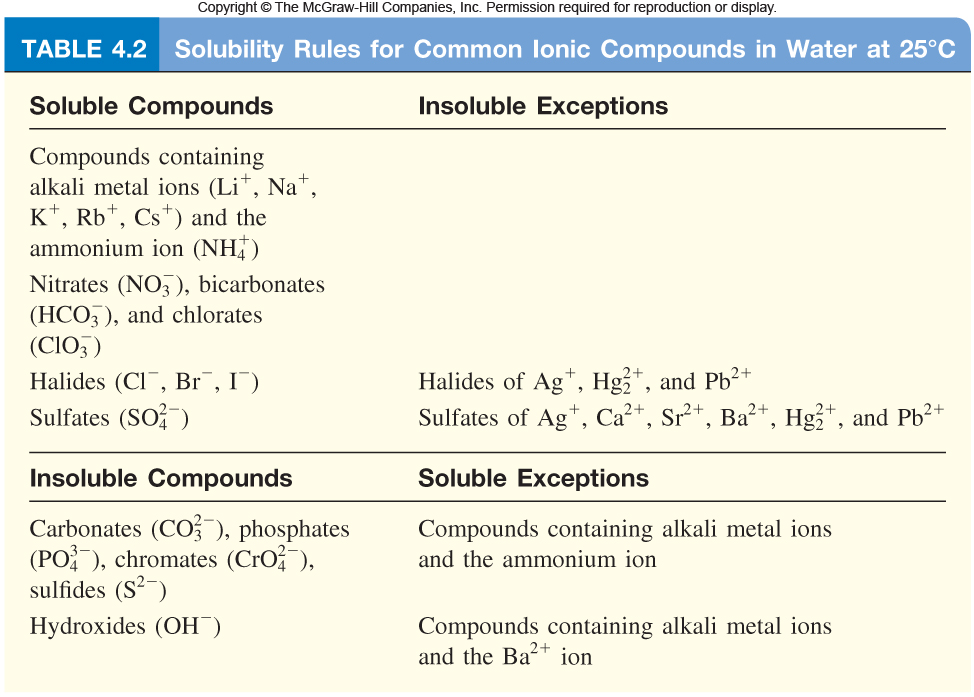
So, start with
SIlver nitrate, or
FInally, copper (II) hydroxyde, or
Here's a link to an interactive version of the solubility rules:

