Question #6126a
1 Answer
Oxidation numbers are assigned to individual atoms and are not written in a chemical reaction unless the element is ionized in a redox reaction or if you are writing a net ionic equation.
Explanation:
Oxidation numbers of an element by itself are not written in a chemical reaction if it is alone or in a compound.
When a compound is ionized, you write the oxidation numbers of the ions (based on the periodic table for elements or the polyatomic ion chart which can be calculated using formal charge).
Coefficients in an ionic equation come from whatever was the subscript in the molecular equation for that element or ion.
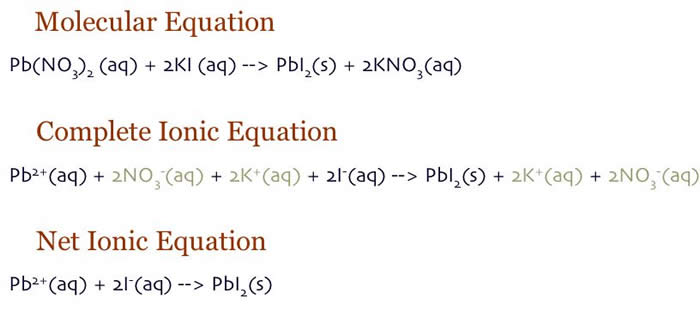
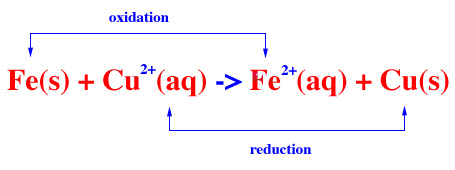
Redox reactions are more complicated. They often use half reactions to track the movement of electrons during electrochemistry.
A simplified redox reaction would be one like this:
Another example would be this:
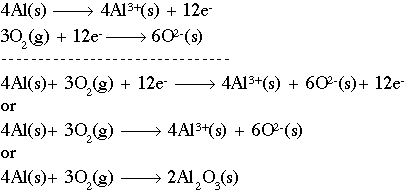
In this example you treat it like a system of equation canceling out items that are on the reactant and product sides of the equation until a final product can be made -- sometimes with no charges in the reactant or product side, sometimes not.
Remember, the coefficient tells you how many of a certain atom at a certain charge you may have and you want the whole reaction to have a charge of zero and each element and compound to have a charge of zero which allows you to obey the Law of Conservation of Atoms, Mass, and Matter.

