Question #06590
1 Answer
The only possible molecular shape for a nonpolar AY₃ is trigonal planar. Trigonal pyramidal and T-shaped molecules are impossible.
There are four possible electron geometries for an AY₃ molecule.
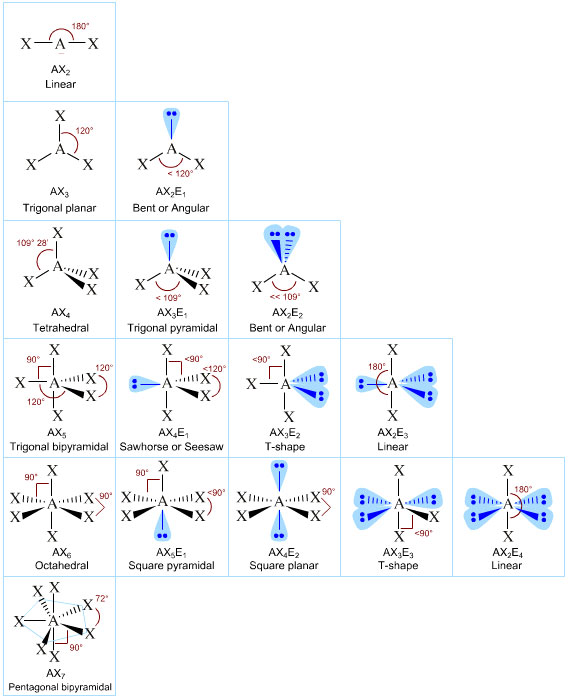
They could be AY₃, AY₃E, AY₃E₂, or AY₃E₃.
AY₃
An AY₃ molecule is trigonal planar.
The A-Y bond dipoles will all cancel, so the molecule will be nonpolar.
AY₃E
An AY₃E molecule is trigonal pyramidal.
The A-Y bond dipoles all have a component pointing in the same direction, and there is nothing to counteract the dipole from the lone pair.
An AY₃E molecule is polar.
AY₃E₂
An AY₃E₂ molecule is T-shaped.
The polar A-Y bond dipoles cancel each other, but it is unlikely that the equatorial A-Y bond will cancel the effect of the lone pairs.
An AY₃E₂ molecule is polar.
AY₃E₃
An AY₃E₃ molecule is T-shaped.
The polar A-Y bond dipoles cancel, and two of the equatorial lone pairs cancel, but it is unlikely that the equatorial A-Y bond will cancel the effect of the equatorial lone pair.
An AY₃E₃ molecule is polar.
So, the three possible molecular shapes are trigonal planar, trigonal pyramidal, and T-shaped, but only the trigonal planar shape is nonpolar.

