Question #2f052
1 Answer
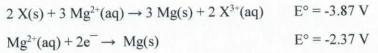

16. d) 1.50 V
2 × [X³⁺(aq) +3e⁻ → X(s)];
3 × [Mg(s) → Mg²⁺(aq) + 2e⁻];
2X³⁺(aq) + 3Mg(s) → 2X(s) + 3Mg²⁺(aq);
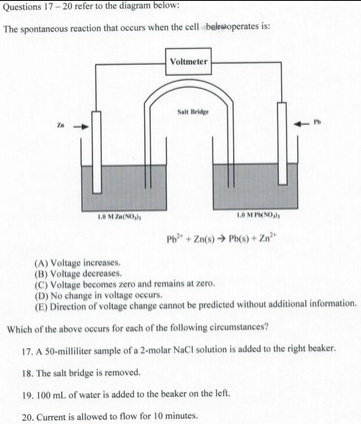
17. PbCl₂ is insoluble. Adding NaCl to the right-hand beaker will cause PbCl₂ to precipitate. This decreases the concentration of Pb²⁺.
According to Le Châtelier's Principle, the position of equilibrium will move to the left. The voltage will decrease.
18. Removing the salt bridge breaks the internal circuit. Ions can no longer flow from one half-cell to the other.
The voltage becomes zero and remains at zero.
19. Adding water to the beaker on the left decreases the concentration of Zn²⁺.
According to Le Châtelier's Principle, the position of equilibrium will move to the right.
The voltage will decrease.
20. If you allow current to flow for 10 min, the concentration of Zn²⁺ will increase, and the concentration of Pb²⁺ will decrease.
Increasing [Zn²⁺] pushes the equilibrium to the left. So does decreasing [Pb²⁺]. The battery is running down. The voltage decreases.

