Buffer or no buffer? #"45 mL"# of #"0.60 M"# #"KF"# reacts with #"25 mL"# of #"0.60 M"# #"HClO"_4# to yield #"HF"# and #"KClO"_4#, all aqueous.
2 Answers
Yes. More info up above.
A buffer is a weak acid/base plus its salt. Like a mixture of acetic acid and sodium acetate.
KF is not a salt of this acid either
A buffer is a weak acid/base plus its soluble conjugate base/acid (which usually works well with an alkali metal as the cation).
The pKa of
In the previous sense,
Yes, this is a buffer with pH = 3.04.
The
The
The
The
The first problem is to figure out how much
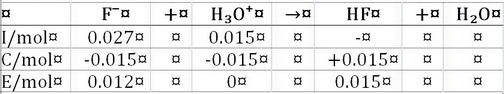
We have a buffer, because we have a solution of a weak acid
To calculate the pH, we use the Henderson-Hasselbalch Equation.
Note that the ratio of the concentrations is the same as the ratio of the moles, because both species are in the same solution.



