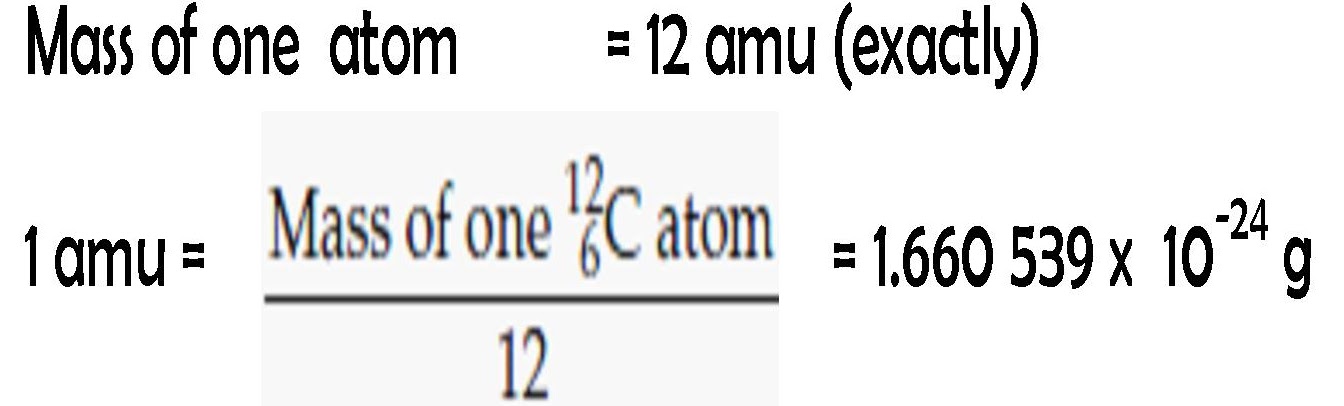Question #cefc7
1 Answer
Apr 10, 2015
One atomic mass unit, or unified atomic mass unit, is defined as 1/12th of the mass of a neutral carbon-12 atom and is equivalent to
Expressed in grams, 1 amu is equal to