Why is a C=N double bond shorter than a C=C double bond?
1 Answer
Jun 4, 2015
Nitrogen has a smaller atomic radius from the periodic table trend of increased
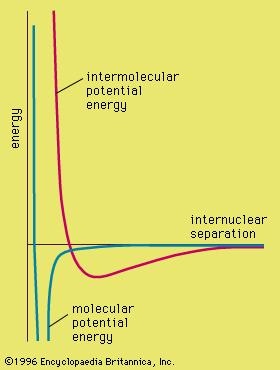
The optimal internuclear distance is shown in a potential energy curve similar to this one:
 http://l.yimg.com/
http://l.yimg.com/

