Question #8fa9a
1 Answer
The only difference between these two scales is where the zero mark is located.
Explanation:
The temperature on the Kelvin scale is simply the temperaure of the Celsius scale plus 273.15.
In other words, if you move the 0 mark on a Celsius scale to 273.15, you get the Kelvin scale.
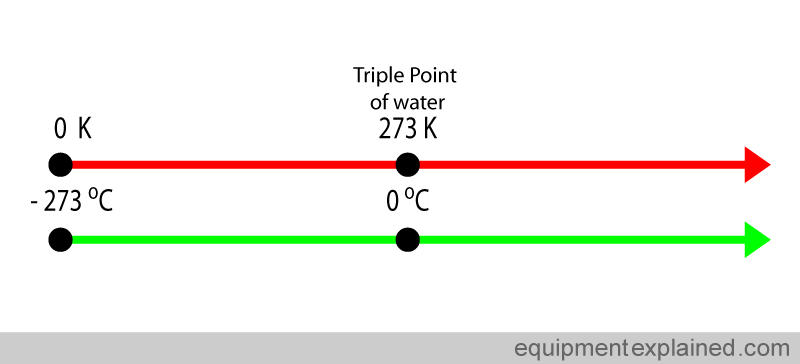
This means that the starting point of the Kelvin scale,
You can use water's freezing and boling temperatures as examples of how the Kelvin scale relates to the Celsius scale.
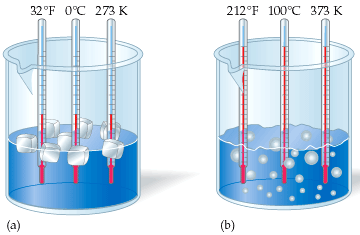
The freezing point of water is
The boling point of water is

