Question #d30a7
1 Answer
You find N by identifying the non-equivalent sets of hydrogen atoms, and M by considering the shapes of the molecules. There are N = 4 isomers and M = 3 fractions.
Explanation:
WARNING: Long answer.
Number of Isomers N
The structure of 2-methylbutane is

There are four different sets of equivalent hydrogens:
- 1 The three H atoms on C-4
- 2 The two H atoms on C-3
- 3 The one H atom on C-2
- 4 The six H atoms on C-3 and the methyl substituent
So there are four possible monochloro substitution products:
1: 1-Chloro-3-methylpentane
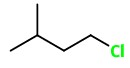
2: 2-Chloro-3-methylpentane
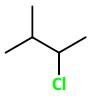
3: 2-Chloro-2-methylpentane
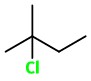
4: 1-Chloro-2-methylpentane
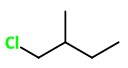
Number of fractions, M
3 has four groups attached to the same carbon atom.
The molecules will have a roughly spherical shape.
They will have little contact with each other and therefore small dipole-dipole and London dispersion forces.
They will have the lowest boiling point.
2 has a chain of four atoms with groups on two different carbon atoms.
The molecules should have better contact with each other and therefore stronger dipole-dipole and London dispersion forces.
2 will have a higher boiling point than 3.
1 and 4 each have a chain if five atoms with only one substituent.
The molecules should have the best contact with each other and therefore the strongest dipole-dipole and London dispersion forces.
1 and 4 should have the highest boiling points, and they should not differ much from each other.
Prediction:
- The lowest boiling Fraction 1 contains 3.
- Fraction 2 contains 2.
- The highest boiling Fraction 3 contains 1 and 4.

