Question #bde65
1 Answer
Because when you add sulfuric acid to water, the protonation of water molecules takes place.
Explanation:
Hydrogen sulfate, or bisulfate ion, as you'll sometimes see it called, cannot act as a conjugate base in solution because sulfuric acid,
In other words, in the first dissociation of sulfuric acid, the equilibrium that forms hydronium ions,
Notice the very large value of the acid dissociation constant,
So, when you place sulfuric acid in water (never do the other way around!), all the sulfuric acid molecules will lose their proton, which is then picked up by water molecules
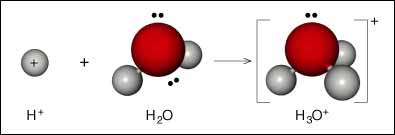
Once this happens, you can consider that the solution no longer contains
However, hydrogen sulfate is a weak acid and thus cannot dissociate completely.
This means that your solution will contain both hydrogen sulfate and bisulfate ions.

