How do you form #"ROMgI"# from #"RMgI"# reacting with ketones?
1 Answer
Oh, you're referring to a Grignard Reagent variant. Normally you should see
While the formation of
Normally, a carbonyl group is a good electrophilic site because basically, it has a high electron density, and it has two p orbitals within its pi bond that have unoccupied space (antibonding atomic orbitals) for electrons in the pi bond to move into (unshaded).
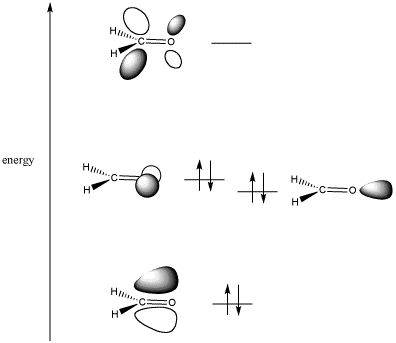
Magnesium, on the other hand, is a strong Lewis acid, so it's a strong electron acceptor. As a result,

