Question #8ede8
1 Answer
Jun 29, 2015
The molar mass of aluminium sulfate is 342.2 g/mol.
Explanation:
Aluminium sulfate is an ionic compound with the formula
It is formed when 2 aluminium cations,
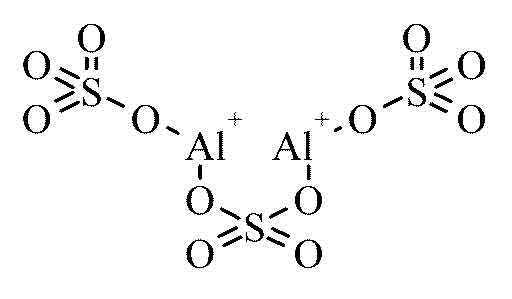
To get the molar mass of the compound you need to add the molar masses of all the atoms that form the aluminium sulfate formula unit.
This means that you have
- two atoms of aluminium -
#2 * "27.0 g/mol"# - three atom of sulfur -
#3 * "32.07 g/mol"# - twelve atoms of oxygen -
#12 * "16.0 g/mol"#
The molar mass of the compound will thus be

