Question #58145
1 Answer
Jul 10, 2015
Your molecule contains 4 pi bonds.
Explanation:
In order to determine how many pi bonds a molecule has, you need to examine its Lewis structure, more specifically you need to look for double or triple bonds.
Keep in mind that
- a single bond is also a sigma bond;
- a double bond contains one sigma and one pi bond;
- a triple bond contains one sigma and two pi bonds;
The Lewis structure for dithionic acid,
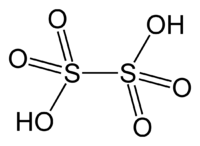
Notice that the molecule has 4 double bonds, all of them between sulfur and oxygen atoms.
SInce each double bond contains one pi bond, the molecule will have a total of 4 pi bonds - one from each of the double bonds.
By comparison, the molecule has 9 sigma bonds - one for each double bond and one for each single bond (the single bonds that exist between the hydrogen and oxygen atoms are not shown).

