Question #5f3ca
1 Answer
Aug 5, 2015
We can try to reduce heat dissipated.
Explanation:
Considering that:
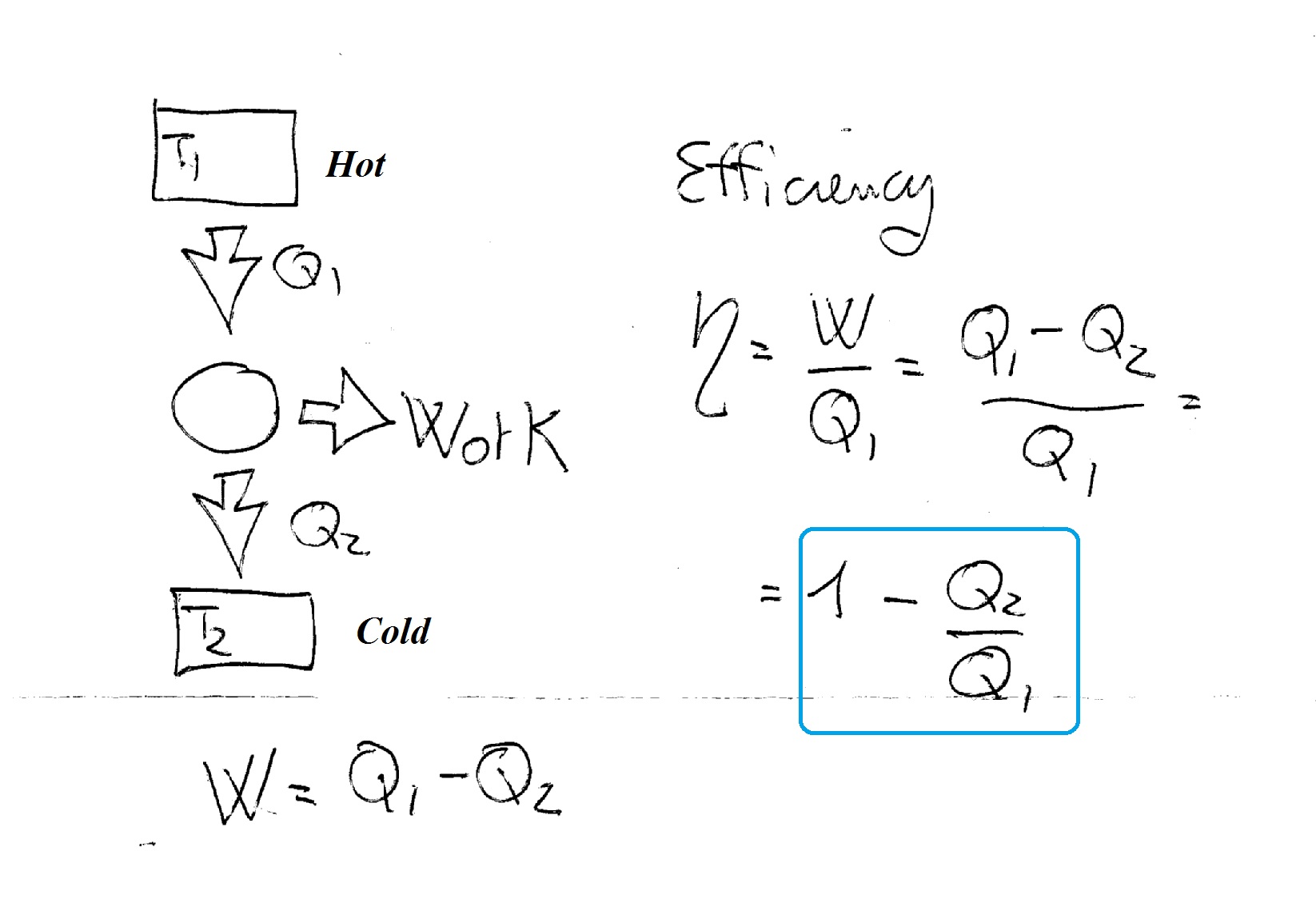
You can see that the efficiency depends heavily upon the heat
If we want to increase efficiency we can try to reduce as much as possible
We will never be able to make it zero though!!! So our efficiency will always be less than 1 (less than 100% which means we will never reach total conversion of heat into work).

