Question #d84c3
1 Answer
The solubility of a gas in a liquid increases with (b) decrease in temperature.
Explanation:
Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature.
There are two direct factors that affect solubility: temperature and pressure.
(a) Increase in temperature

(from www.lakelandschools.org)
According to Kinetic Molecular Theory, as the temperature increases, the gas molecules move faster and are then able to escape from the liquid.
The solubility of the gas decreases.
(b) Decreasing the temperature
Decreasing the temperature means that the molecules have less kinetic energy and are less able to escape into the atmosphere.
The solubility of the gas increases.
We can see this effect in the solubility chart for ammonia.
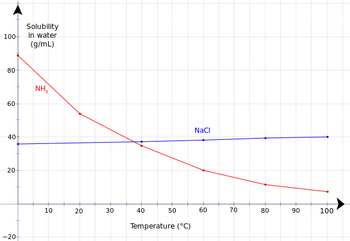
(from en.wikibooks.org)
The solubility of ammonia is 35 g/100 mL of water at 40 °C but it increases to 54 g/100 mL at 20 °C.
(c) Decreasing the Pressure.
In a closed container, molecules of the gas are moving into the gas phase as fast as they are returning to the liquid phase.
If you decrease the pressure, more molecules will move into the gas phase until equilibrium is restored.
The solubility of the gas will decrease.
(d) Increasing the volume of solvent.
The solubility of ammonia in water is 54 g/100 mL. If you add another 100 mL of water, the solution will hold 108 g of ammonia.
The solution holds more ammonia, but the solubility hasn't changed.
It is still 54 g/100 mL.


